Editors and reviewers of the journal Green Chemistry have highlighted a new study from the University of Texas at Arlington investigating how to make common chemical techniques more environmentally friendly as one of its “hot” articles for 2023.
UTA scientists led by Daniel W. Armstrong, the Welch Distinguished Professor of Chemistry and Biochemistry, found that using carbonated water in chromatography makes this relatively common chemical technique more environmentally benign.
A technique that works by taking a mixture and separating it to examine the individual components, chromatography is widely used to test athletes’ urine for performance-enhancing drugs, analyze crime scene evidence such as blood and cloth, test the ingredients in food, or measure the amount of alcohol in drinks, among many other uses. A single chromatograph produces about a liter of liquid waste, with some major pharmaceutical companies operating more than 1,000 chromatographic studies per day.
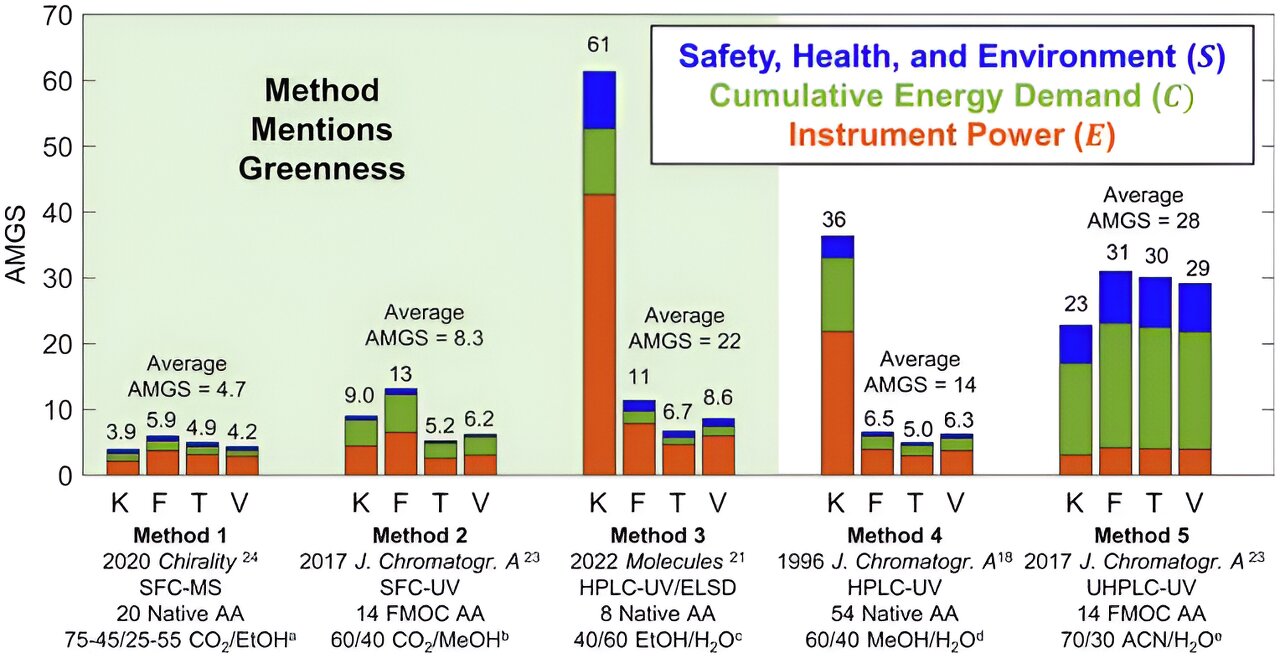
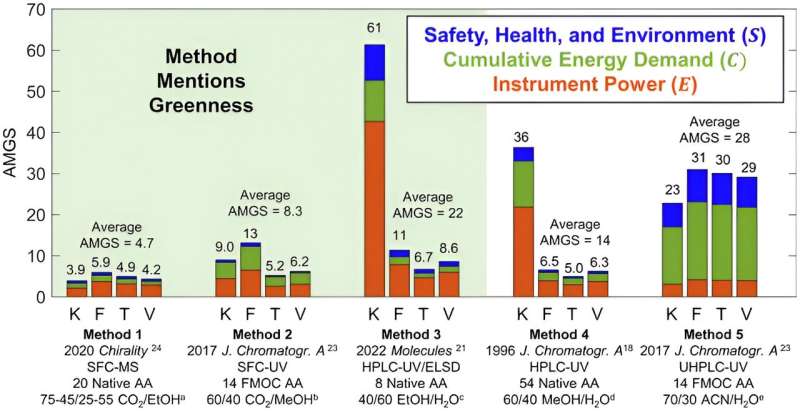
Using carbonated water in chromatography can reduce the technique’s Analytical Method Greenness Score (AMGS). The smaller the score, the more environmentally friendly the process is, Armstrong said.
“Our research shows that the use of simple carbonated water plus minimal mathematical processing and optimal column geometries produces the lowest AMGS scores yet reported,” Armstrong said. “This shows that switching to carbonated liquids instead of other liquids when possible will help make the process of chromatography safer for the environment.”
The team also found that using carbonated liquids is just as fast and efficient as other liquids used in chromatography.
“Using 38 amino acids as a test class of molecules, the utility of carbonated liquids as a green alternative was presented at speeds, efficiencies and resolutions never reported,” Armstrong said. “Future work will involve applying what we learned regarding carbonated liquids in chromatography to other methodologies, such as mass spectrometry.”
This study also corrected the original AMGS equation and extended it to cover more realistic separations including chiral amino acids. The research also noted that this same approach would be useful for NASA, which has special interests in developing lightweight and small instruments for extraterrestrial in situ chiral/achiral chemical analysis.
Co-authors include M. Farooq Wahab, a research engineering scientist, as well as two students: Troy T. Handlovic and Bailey C. Glass. Handlovic received a Bachelor of Science in chemistry and Master of Science in pharmaceutical chemistry from Fairleigh Dickinson University in Madison, New Jersey, before coming to UTA to pursue his doctoral degree. Glass, who is from Arlington, is a sophomore undergraduate research assistant studying biochemistry with plans to continue studying chemistry in graduate school.
More information:
Troy T. Handlovic et al, Optimization of analytical method greenness scores: a case study of amino acid enantioseparations with carbonated aqueous systems, Green Chemistry (2023). DOI: 10.1039/D3GC03005A
Provided by
University of Texas at Arlington
Citation:
Using carbonated water in chromatography makes for a greener process, new study finds (2023, December 20)
retrieved 20 December 2023
from https://phys.org/news/2023-12-carbonated-chromatography-greener.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.





