Targeted drugs aim to pinpoint the exact location in the body where diseased tissue is located and where the medicine is required. The manifold benefits of administering a targeted drug include heightened efficacy, as the drug is meticulously designed for specificity, thereby reducing side effects, and minimizing damage to healthy tissue. Consequently, this approach enhances the patient’s quality of life during treatment.
Oligonucleotides (ONs), specifically designed short chains of DNA or RNA, have emerged as a crucial tool with immense potential in personalized medicine. These therapeutic ONs are already in use for conditions, such as certain types of muscular dystrophy and liver disease, which conventional drugs cannot address.
Depending on the type, ONs can function by, preventing or changing the production of a protein in the cell, particularly beneficial in diseases caused by the overproduction of a specific protein.
Peptide conjugates as a precise drug delivery solution
However, a persistent challenge lies in precisely delivering therapeutic ONs to a broader range of tissues where treatment of diseased cells is needed. Researchers have attempted to overcome this hurdle by attaching small, specific protein markers to these ONs, known as peptide conjugates, recognizable by the diseased cells.
One such peptide is glucagon-like peptide-1 (GLP1), which has been used to specifically target therapeutic ONs to the pancreas. This peptide is the natural analog of the diabetes and obesity drug Semaglutide sold as Ozempic, Rybelsus and Wegovy.
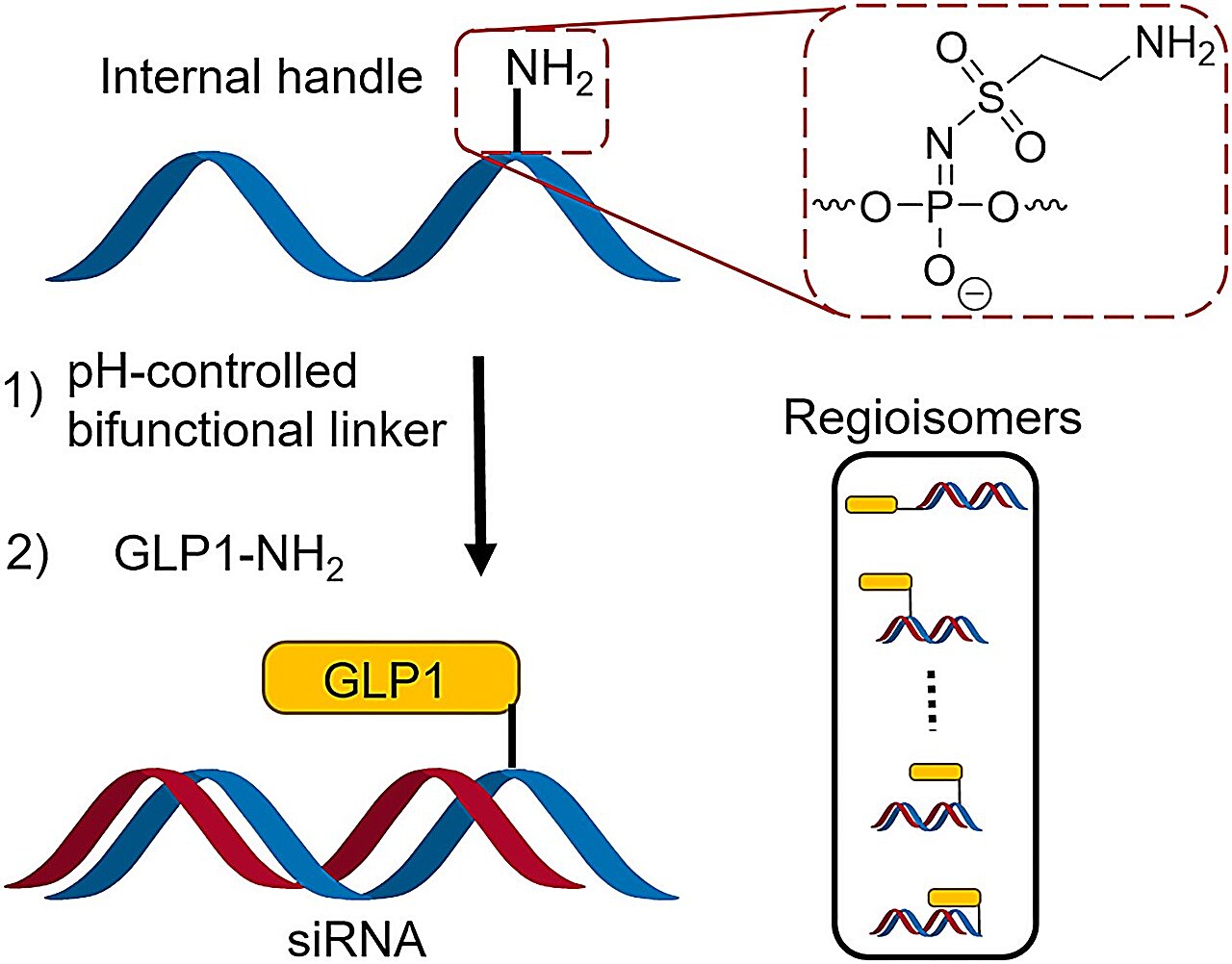
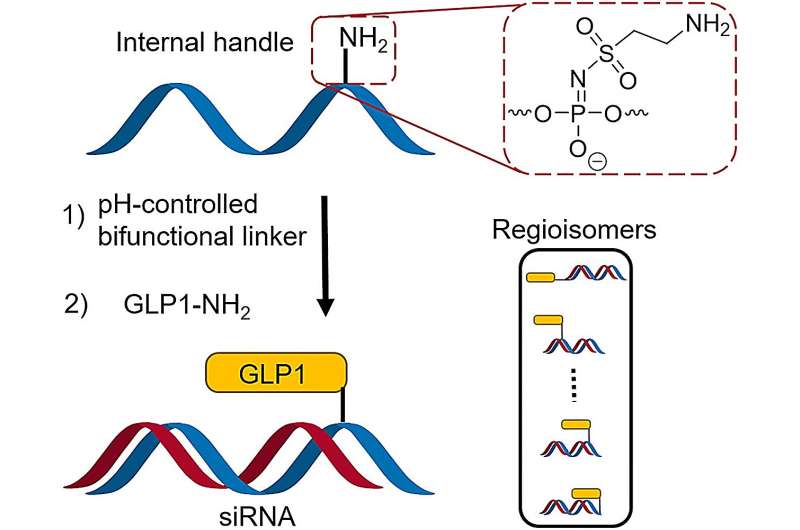
The positioning of chemical modifications on the therapeutic ON is crucial for the clinical success of this class of drugs. Likewise, the placement of GLP1 in peptide-ON conjugates could be of absolute importance. However, placing the ligand inside the ON sequence has, until now, required specialized and costly ON building blocks.
Development of peptide conjugates without expensive building blocks
In close collaboration with Novo Nordisk, Professor Kurt Gothelf and his research group have now devised a method to simplify the construction of an entire library of therapeutic ON—peptide conjugates. As a central part of the collaboration, iNANO Ph.D. student Jakob M. Smidt visited Specialist Lennart Lykke at Novo Nordisk in Måløv.
The expertise led to a highly successful collaboration with novel discoveries that are beneficial for researchers in the pharmaceutical industry and at universities. In this collaboration, the researchers have discovered a synthesis method for ON conjugates that incorporates built-in handles and a special linker, enabling easy linkage of ONs to a peptide marker by adjusting the pH.
The noteworthy aspect of this method is the elimination of the need for specialized and expensive ON building blocks to integrate peptides into the oligonucleotide sequence.
Potential for effective medicine with multiple functions
The significance of this method lies in its streamlining of the process, making the production of these conjugates more accessible and cost-effective. This breakthrough holds the potential to produce therapeutic ONs with multiple functions, paving the way for more effective drugs.
By joining the expertise in ON chemistry, including novel functionalization technology, of the Gothelf lab together with the peptide science legacy of Novo Nordisk, new and robust conjugation chemistry has emerged. The method has enabled insights into the structure-activity relationship of ON conjugates and is thus a very important scientific contribution to understanding this promising class of therapeutic molecules.
This work is the product of a fruitful public-private collaboration where knowledge and new ideas have openly been shared and discussed with the common ambition to make a scientific impact and potentially make a difference to people living with disease.
Gothelf envisions a future where this method directs ON-based drugs to specific tissues in the body. The development of this method marks a significant stride towards more effective and targeted drugs, indicating the potential for customized therapeutic ONs to deliver drugs precisely to the intended location in the body.
More information:
Jakob Melgaard Smidt et al, Synthesis of peptide–siRNA conjugates via internal sulfonylphosphoramidate modifications and evaluation of their in vitro activity, Nucleic Acids Research (2023). DOI: 10.1093/nar/gkad1015
Provided by
Aarhus University
Citation:
New chemical method advances toward targeted RNA medicine (2023, December 13)
retrieved 13 December 2023
from https://phys.org/news/2023-12-chemical-method-advances-rna-medicine.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.


